Practice point
Approaches to detecting tuberculosis in children and youth
Posted: Dec 14, 2018 | Updated: Jul 16, 2024 | Addendum: Jul 2, 2024
Principal author(s)
Nicole Le Saux; Canadian Paediatric Society, Infectious Diseases and Immunization Committee
Updated by: Michelle Barton MD, Ari Bitnun MD
Abstract
This practice point provides a framework for initiating investigation in children suspected of having infection with Mycobacterium tuberculosis (Mtb). Some areas in Canada have a high burden of tubercular disease, with Indigenous populations being most at risk. Tuberculosis (TB) can present either as an acute or subacute illness and both primary or reactivation infection can cause pulmonary or multisystem disease. Tuberculin skin tests and interferon-γ release assays can be used to support a suspected diagnosis but may be negative in 10% to 30% of TB disease cases. TB elimination in Canada is possible but requires improving social determinants of health, one of the major factors contributing to the spread of TB in populations at risk.
Keywords: Bacille-Calmette-Guérin; Break of contact; Latent tuberculosis infection; Mycobacterium tuberculosis; Tuberculosis
This practice point highlights when to suspect tuberculosis (TB) in children and youth and how to initiate investigations. Updated guidance from the 8th edition of the Canadian TB Standards, a comprehensive review of the epidemiology and management of paediatric TB in Canada published in 2022, is reflected in this document[1].
TB disease must always be considered in Indigenous children and youth who are living or have lived in areas with risk factors (i.e., overcrowded, poorly ventilated houses that exist in many Indigenous communities where TB has been endemic for decades). TB should also be considered both in foreign-born children and children of foreign-born individuals from countries with a high burden of TB (http://www.stoptb.org/countries/tbdata.asp). The incidence of TB between 2010 and 2020 in Canada overall was 4.5 to 4.9 per 100,000 population but peaked in 2012 at 251 per 100,000 population. By 2019, the incidence had decreased to 188.7 per 100,000, then to 70 per 100,000 in the first year of the pandemic[1]-[3].
Latent tuberculosis infection (LTBI) implies that Mycobacterium tuberculosis (Mtb) bacteria are ‘dormant’, such that the infected individual is asymptomatic and non-infectious[4]. By contrast, TB ‘disease’ indicates that there is bacterial replication, such that the individual with infection is typically symptomatic.
Bacille-Calmette-Guérin (BCG) is a live, attenuated vaccine given at birth to infants residing in Canadian jurisdictions considered to have high rates of smear-positive TB[5]. BCG is contraindicated for infants with a family history of immunodeficiency or who are suspected of being immunodeficient[6][7].
Tuberculosis (TB) exposure and infection
Exposure commonly occurs through breathing household air that contains suspended aerosolized droplets of Mtb, usually from cough expectorations by an adult or adolescent (e.g., parents, grandparents, siblings). Exposure can also occur during sports events or community gatherings and in school or child care settings.
Source cases with cavitary disease are highly infectious and risk for transmission increases when sputum has a high density of Mtb (i.e., when bacilli are seen on the sputum smear)[8]-[14]. Table 1 illustrates some key points on history, along with their corresponding clinical significance.
Mycobacterium tuberculosis (Mtb) infection
Primary infection is established when Mtb bacilli are inhaled and replicate in the pulmonary alveoli. There, they are engulfed by macrophages, leading to the formation of granulomas and causing hilar or mediastinal lymphadenopathy. The parenchymal site is a Ghon focus, whereas Ghon complex includes adjacent hilar lymphadenopathy. This initial infection may lead to spread of Mtb to other lymph nodes and/or hematogenous dissemination to other organs. Over time, granulomas may calcify, but Mtb is likely to remain viable though dormant[4].
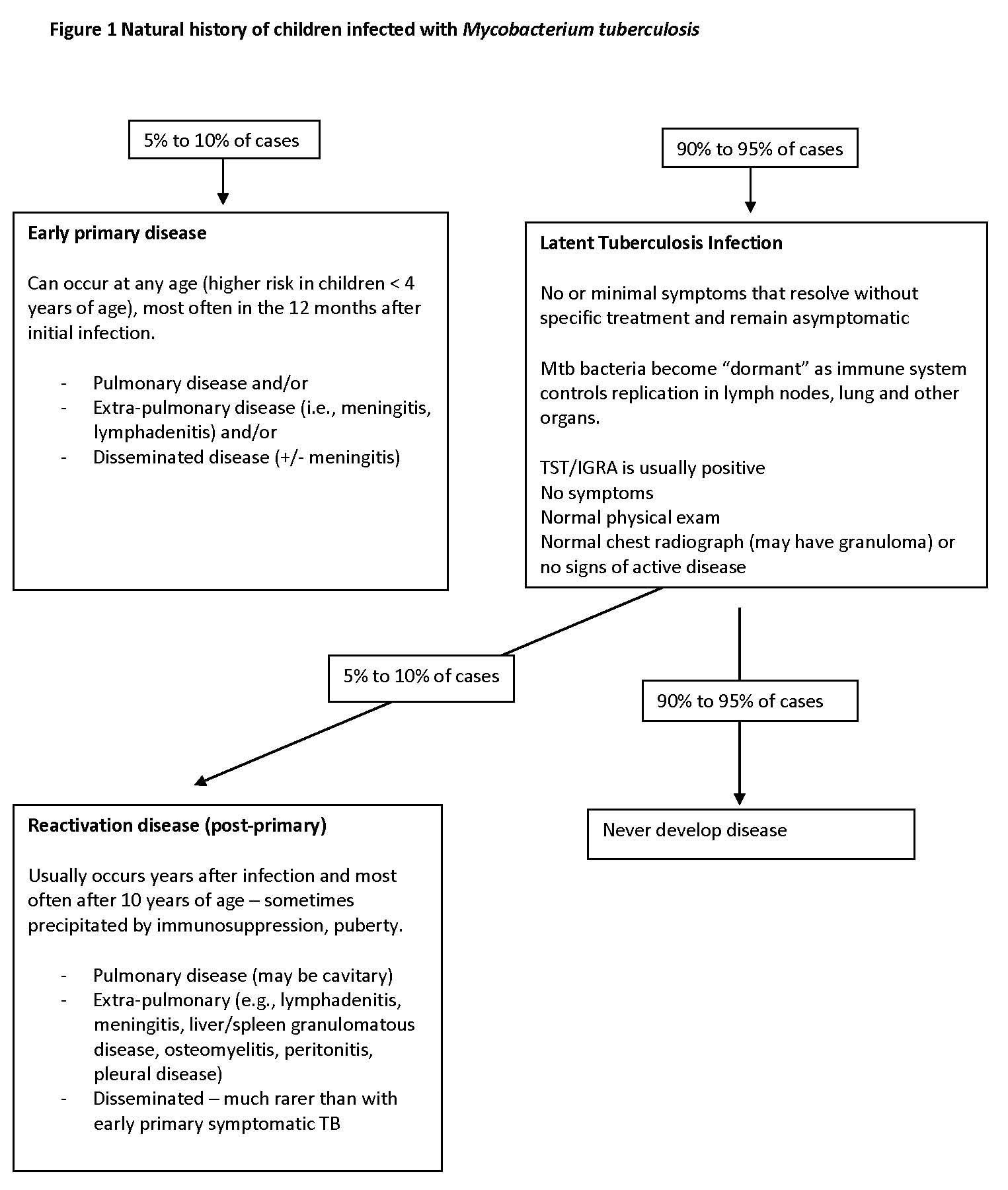
Among children who have primary infection with Mtb, 90% to 95% experience minimal symptoms or are asymptomatic. They do not develop clinical disease and carry viable though dormant bacteria. This state of infection is commonly referred to as LTBI. Figure 1 describes the natural history of children infected with Mtb.
|
|
Early primary TB disease
Five to ten per cent of children infected with Mtb present with primary disease—either pulmonary or extra-pulmonary—with the vast majority (an estimated 90%) presenting clinically within one year after primary infection[15]. Infants and children <5 years old are especially vulnerable.
Pulmonary
Pulmonary symptoms may be acute, mimicking pneumonia, or indolent, with wheezing or subacute symptoms[15]. Both lateral and postero-anterior radiographs are required for diagnosis because hilar lymphadenopathy is often best seen on a lateral view. Radiographs typically show focal pneumonitis or subtle ‘ground glass’ opacities, with (usually) hilar, mediastinal or subcarinal lymphadenopathy[16]-[18]. Chest computed tomography (CT), with or without contrast, is more sensitive and specific for detecting pulmonary TB, but this technique involves radiation and, possibly, sedation[19]-[21].
Disseminated disease or extra-pulmonary disease
When the immune system fails to contain early pulmonary infection, disseminated disease ensues. Infants are at particularly high risk[22].
In this context, constitutional symptoms may predominate, including weight loss, poor feeding, prolonged or recurrent fever, and lethargy or irritability. Syndromic presentations include pneumonia, meningitis, osteoarticular infections or sepsis unresponsive to antibiotics. Multiple organs can be involved, especially the lung, brain, retina, liver, spleen, bone marrow and muscle, making diagnosis challenging. Miliary nodules or a ‘tree-in-bud’ pattern on chest radiograph are typical, but a diffuse alveolar pattern or acute respiratory distress syndrome can also occur[16][17]. In meningitis, the cerebrospinal fluid (CSF) typically shows pleocytosis, with lymphocytic predominance. Magnetic resonance imaging (MRI) may be helpful. Because neonatal (congenital) TB is also commonly disseminated, diagnosis is often delayed when maternal diagnosis is missed.
Reactivation TB disease
An estimated 5% to 10% of children with LTBI, eventually reactivate the dormant infection and present with signs and symptoms of disease. Previously known as “post-primary” disease, reactivation can occur many years after the primary infection.
Although reactivation can occur in healthy young children, it occurs most commonly in adults or adolescents. Aside from likely host genetic factors, other risk factors include immunosuppression (e.g., human immunodeficiency virus (HIV) infection or diabetes), malnutrition or medications (especially steroids or biologics)[15][23]. Children and adolescents with pulmonary reactivation may present with ‘adult-type’ cavitary disease or infiltrates, often in the apical and upper lung zones. Other manifestations include tuberculous pleural effusions, lymphadenitis, central nervous system (CNS) tuberculous lesions, hepatic or splenic abscesses, osteoarticular infections, and (less commonly) disseminated disease.
Laboratory tests
When tuberculosis is suspected clinically, obtaining cultures is a vital next step. Cultures lead to a definitive diagnosis, permit drug resistance testing and may allow for molecular fingerprinting to link cases.
Specimens for microbiology
Sputum production can be induced by inhalation of hypertonic saline. In children who cannot expectorate sputum, fasting gastric aspirates obtained on three consecutive mornings are useful for culture[18][24]. The Curry International Tuberculosis Centre website describes the appropriate procedure: (http://www.currytbcenter.ucsf.edu/products/pediatric-tuberculosis-guide-gastric-aspirate-procedure). Bronchoscopy may be required to obtain specimens.
Appropriate specimens from fluid or tissues should be sent in sterile containers (without formalin) to the laboratory for acid-fast bacilli (AFB) stains and culture, with possible nucleic acid amplification testing (NAAT). Swabs are not appropriate samples. Testing may be referred to accredited laboratories and protocols vary[25]. Specimens vary in quality, such that a negative smear or culture should not exclude a diagnosis of TB, when clinically compatible.
All patients with TB disease require serology for HIV.
Tuberculin skin testing and interferon-gamma release assays (IGRA)
Tuberculin is a purified protein derivative (PPD) from heat-inactivated Mtb. When injected intradermally as a tuberculin skin test (TST), a type IV hypersensitivity reaction (wheal) occurs if the recipient has been infected with Mtb or if they have cross-reactive antigens from non-tuberculous mycobacteria (NTM) or from BCG vaccine[26][27]. Cut-offs for TST induration indicative of possible infection are detailed in the Canadian Tuberculosis Standards, but generally are ≥5 mm induration for individuals who are immunocompromised and for contacts of cases and ≥10 mm for others. Of individuals who received BCG at or soon after birth, only 1% will have a TST of ≥10 mm after 10 years of age. Therefore, a TST of this size usually should not be attributed to past receipt of BCG vaccine[28]. Infants or young children with suspected TB disease may even have 0 mm or <5 mm induration.
The interferon-gamma release assays QuantiFERON-TB Gold In-Tube and the T-Spot. TB are in-vitro blood tests that evaluate immune response by measuring the release of interferon-gamma by T-cells in response to antigens specific for Mtb. There is no cross-reactivity with BCG and minimal reactivity with nontuberculous mycobacteria (NTM). Therefore, the specificity is >95%, compared with 60% for a TST[29].
Both IGRA and TST can yield false-negative results in the setting of immunosuppression. They also may be negative in 10% to 30% children with TB disease, especially miliary disease, while IGRA can also signal falsely positive from a recent TST[29]. Neither test differentiates LTBI from active disease because in both situations, the child or youth is infected[26][27]. In children ≥2 years old, TST and IGRA were thought to have similar sensitivity and specificity for diagnosis of LTBI. However, IGRA is more specific than TST, especially in individuals who have received BCG, thus reducing the need for further testing and treatment if IGRA is negative. There are few studies of children <2 years old at the present time, but a TST is possibly more sensitive than IGRA. Since this group is at higher risk for disease progression, the more sensitive TST is currently recommended over IGRA. Using clinical judgement on a case-by-case basis is paramount, however[27][30]. Provincial/territorial health authorities may or may not pay for IGRA tests, depending on indications.
Public health implications
The high incidence rate of TB among Indigenous peoples in Canada is a serious public health issue. Consideration of current and historical social determinants of health are critical to providing quality, culturally competent care and to addressing TB at the policy level.
Timely prophylaxis of children and adults with LTBI is essential for preventing progression to active disease and subsequent transmission of Mtb. Investigation and treatment of symptomatic contacts of TB cases should be expedited to limit further transmission. TB disease must be reported to local public health authorities within 48 h of diagnosis to prevent spread. Typically, when respiratory secretions from an index case smear positive, the individual is isolated in hospital or at home until three sputum specimens smear negative or, if initial smears are negative, after a full 2 weeks of directly observed therapy has been administered.
When the index case is a child with primary disease, the focus of contact tracing is to identify the source, which is usually an adult or youth because young children with primary disease are usually non-infectious[31].
When a child or youth is identified as a contact of an index case, conducting a history and physical exam, requesting chest radiographs and performing an initial TST are essential steps. Obtaining the index case’s drug sensitivities is also required. Child contacts <5 years of age with an initial TST of <5 mm should receive preventive prophylaxis (also known as ‘window prophylaxis’, with one TB drug), using a drug that has been identified as effective for treating the source case strain. A second TST is done at 8 to 10 weeks following last contact (sometimes termed “break of contact”(BOC)), while the index case was still infectious. A child ≥5 years old with an initial TST <5 mm in size needs a BOC TST 8 to 10 weeks later, although window prophylaxis is not recommended in this age group.
Child contacts <5 years old, with a BOC TST <5 mm in size at 8 to 10 weeks, can then have window prophylaxis discontinued. Child contacts ≥5 years who have no symptoms and whose physical exam and chest radiographs appear normal, with an initial or BOC TST of ≥5 mm, should be treated for latent infection[28][32]-[34].
Treatment
To prevent reactivation, individuals with LTBI are usually treated with isoniazid, rifampin or rifapentin/isoniazid, in collaboration with local public health authorities[32]-[35]. Patients with tuberculosis disease are usually started on a four-drug therapeutic regimen in accordance with the Canadian Tuberculosis Standards and in consultation with specialists in infectious diseases and/or respirology[36][37].
Addendum
The American Academy of Pediatrics’ Redbook 2024 recommends using either interferon-gamma release assays (IGRA) or tuberculin skin testing (TST) for TB screening in all children, even those under 2 years of age. Their recommendation is based on available data indicating similar test performance results for IGRA in children younger than 2 years of age and those 2 years of age and older. However, both tests are needed for infants with congenital TB due to the low sensitivity of either test in such infants. When congenital TB is suspected in young infants with sepsis or pneumonia unresponsive to antibiotics (e.g., whose mothers have risk factors for TB or known genital TB), both IGRA and TST should be performed to increase sensitivity[38].
Acknowledgements
This practice point was reviewed by the First Nations, Inuit and Métis Health and Community Paediatrics Committees of the Canadian Paediatric Society, as well as the CPS Caring for Kids New to Canada Task Force. Special thanks are also due to Dr. Ian Kitai, tuberculosis specialist with the division of infectious diseases at the Hospital for Sick Children, in Toronto, Ontario.
CPS INFECTIOUS DISEASES AND IMMUNIZATION COMMITTEE
Members: Michelle Barton-Forbes MD; Sean Bitnun MD; Natalie A Bridger MD; Shalini Desai MD (past member); Michael Forrester MD (Resident member); Ruth Grimes MD (Board Representative); Nicole Le Saux MD (Chair); Otto G Vanderkooi MD
Liaisons: Upton D Allen MBBS, Canadian Pediatric AIDS Research Group; Tobey Audcent MD, Committee to Advise on Tropical Medicine and Travel (CATMAT), Public Health Agency of Canada; Carrie Byington MD, Committee on Infectious Diseases, American Academy of Pediatrics; Fahamia Koudra MD, College of Family Physicians of Canada; Marc Lebel MD, IMPACT (Immunization Monitoring Program, ACTIVE); Jane McDonald MD, Association of Medical Microbiology and Infectious Disease Canada; Dorothy L Moore MD, National Advisory Committee on Immunization (NACI); Howard Njoo MD, Public Health Agency of Canada
Consultant: Noni E MacDonald MD
Principal author: Nicole Le Saux MD
Updated by: Michelle Barton MD, Ari Bitnun MD
References
- Dwilow R, Hui C, Kakkar F, Kitai I. Chapter 9: Pediatric Tuberculosis. Can J Resp Crit Care Sleep Med 2022;6(Suppl 1):129-48. doi: 24745332.2022.2043055.
- Kitai I, Morris SK, Kordy F, Lam R. Diagnosis and management of pediatric tuberculosis in Canada. CMAJ 2017;189(1):E11–6.
- Mounchilia A, Pereraa R, Leeb RS, Njooa H, Brooks J. Chapter 1: The epidemiology of Tuberculosis in Canada; Canadian Tuberculosis Standards, 8th edition. Can J Resp Crit Care Sleep Med. 2022;6(Suppl 1):8-21.
- Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med 2015;37(22)2:2127–35.
- Fletcher HA. Sleeping Beauty and the story of the Bacille Calmette-Guérin vaccine. MBio 2016;7(4):pii;e01370-16.
- Venkataraman A, Yusuff M, Liebeschuetz S, Riddell A, Prendergast AJ. Management and outcome of Bacille Calmette-Guérin vaccine adverse reactions. Vaccine 2015;33(41):5470–4.
- Marciano BE, Huang CY, Joshi G, et al. BCG vaccination in patients with severe combined immunodeficiency: Complications, risks, and vaccination policies. J Allergy Clin Immunol 2014;133(4):1134–41.
- Jones-López EC, Namugga O, Mumbowa F, et al. Cough aerosols of Mycobacterium tuberculosis predict new infection: A household contact study. Am J Respir Crit Care Med 2013;187(9):1007–15.
- Adam HJ, Guthrie JL, Bolotin S, et al. Genotypic characterization of tuberculosis transmission within Toronto’s under-housed population, 1997-2008. Int J Tuberc Lung Dis 2010;14(10):1350–3.
- Logan JJ, Jolly AM, Blanford JI. The sociospatial network: Risk and the role of place in the transmission of infectious diseases. PLoS One 2016;11(2):e0146915.
- Rossi C, Zwerling A, Thibert L, et al. Mycobacterium tuberculosis transmission over an 11-year period in a low-incidence, urban setting. Int J Tuberc Lung Dis 2012;16(3):312–8.
- Yeo IK, Tannenbaum T, Scott AN, et al. Contact investigation and genotyping to identify tuberculosis transmission to children. Pediatr Infect Dis J 2006;25(11):1037–43.
- Patterson B, Morrow CD, Kohls D, Deignan C, Ginsburg S, Wood R. Mapping sites of high TB transmission risk: Integrating the shared air and social behaviour of TB cases and adolescents in a South African township. Sci Total Environ 2017;583:97–103.
- Singh M, Mynak ML, Kumar L, Mathew JL, Jindal SK. Prevalence and risk factors for transmission of infection among children in household contact with adults having pulmonary tuberculosis. Arch Dis Child 2005;90(6):624–8.
- Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med 2012;367(4):348–61.
- Hashemian SM, Tabarsi P, Karam MB, et al. Radiologic manifestations of pulmonary tuberculosis in patients of intensive care units. Int J Mycobacteriol 2015;4(3):233–8.
- Nachiappan AC, Rahbar K, Shi X, et al. Pulmonary tuberculosis: Role of radiology in diagnosis and management. Radiographics 2017;37(1):52–72.
- Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017;64(2):111–5.
- Swaminathan S, Raghavan A, Datta M, Paramasivan CN, Saravanan KC. Computerized tomography detects pulmonary lesions in children with normal radiographs diagnosed to have tuberculosis. Indian Pediatr 2005;42(3):258–61.
- Golub JE, Bur S, Cronin WA, et al. Delayed tuberculosis diagnosis and tuberculosis transmission. Int J Tuberc Lung Dis 2006;10(1):24–30.
- Veedu PT, Bhalla AS, Vishnubhatla S, et al. Pediatric vs adult pulmonary tuberculosis: A retrospective computed tomography study. World J Clin Pediatr 2013;2(4):70–6.
- Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: A critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004;8(4):392–402.
- Salgame P, Geadas C, Collins L, Jones-López E, Ellner JJ. Latent tuberculosis infection--Revisiting and revising concepts. Tuberculosis (Edinb) 2015;95(4):373–84.
- Hatherill M, Hawkridge T, Zar HJ, et al. Induced sputum or gastric lavage for community-based diagnosis of childhood pulmonary tuberculosis? Arch Dis Child 2009;94(3):195–201.
- Pai M, Minion J, Jamieson F, Wolfe J, Behr M. Diagnosis of active tuberculosis and drug resistance: www.canada.ca/en/public-health/services/infectious-diseases/canadian-tuberculosis-standards-7th-edition/edition-22.htmlh (Accessed December 4, 2017).
- Laurenti P, Raponi M, de Waure C, Marino M, Ricciardi W, Damiani G. Performance of interferon-ƴ release assays in the diagnosis of confirmed active tuberculosis in immunocompetent children: A new systematic review and meta-analysis. BMC Infect Dis 2016;16:131.
- Lowenthal P, Barry PM, Flood J. High discordance between pre-US and post-US entry tuberculosis test results among immigrant children. Is it time to adopt interferon gamma release assay for preentry tuberculosis screening? Ped Inf Dis J 2016;35(3):231-6.
- Pai M, Kunimoto D, Jaimieson F, Menzies D. Diagnosis of latent tuberculosis infection: www.canada.ca/en/public-health/services/infectious-diseases/canadian-tuberculosis-standards-7th-edition/edition-22.htmlh (Accessed December 4, 2017).
- Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014;27(1):3–20.
- Yun KW, Kim YK, Kim HR, Lee MK, Lim IS. Usefulness of interferon- ƴ release assay for the diagnosis of latent tuberculosis infection in young children. Korean J Pediatr 2016;59(6):256–61.
- Rea E, Rivest P. Contact follow-up and outbreak management in tuberculosis control: www.canada.ca/en/public-health/services/infectious-diseases/canadian-tuberculosis-standards-7th-edition/edition-22.htmlh (Accessed December 4, 2017).
- Diallo T, Adjobimey M, Ruslami R, et al. Safety and side effects of rifampin versus isoniazid in children. N Engl J Med 2018;379(5):454–63.
- Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med 2018;379(5):440–53.
- Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011;365(23):2155–66.
- Menzies D, Alvarez GG, Khan K. Treatment of latent tuberculosis infection: www.canada.ca/en/public-health/services/infectious-diseases/canadian-tuberculosis-standards-7th-edition/edition-22.htmlh (Accessed December 4, 2017).
- Kitai I, Demers A. Pediatric tuberculosis: www.canada.ca/en/public-health/services/infectious-diseases/canadian-tuberculosis-standards-7th-edition/edition-22.htmlh (Accessed December 4, 2017).
- Menzies D, Elwood K. Treatment of tuberculosis disease: www.canada.ca/en/public-health/services/infectious-diseases/can
- American Academy of Pediatrics. Tuberculosis. In: Kimberlin DW, Banerjee R, Barnett ED, Lynfield R, Sawyer MH, eds. Red Book: 2024 Report of the Committee on Infectious Diseases. Itasca, IL: AAP; 2024.
Disclaimer: The recommendations in this position statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. Internet addresses are current at time of publication.
Last updated: Oct 8, 2024