Position statement
Cow’s milk protein allergy in infants and children
Posted: Jun 25, 2024
Principal author(s)
Pushpa Sathya MD, Tanis R. Fenton RD, PhD; Canadian Paediatric Society, Nutrition and Gastroenterology Committee
Paediatr Child Health 29(6):382–388. 
Abstract
Cow’s milk protein allergy (CMPA) is an immune-mediated reaction to cow’s milk proteins, which can involve multiple organ systems including the gastrointestinal tract. Immunoglobulin E (IgE)-mediated response results in rapid onset of allergic symptoms that are easily recognizable. However, delayed (i.e., non-IgE/cell-mediated), or mixed (IgE- and cell-mediated) reactions produce a host of symptoms that overlap with other conditions and vary widely in onset and severity. Determining whether symptoms represent immune-mediated CMPA, non-immunologic reaction to cow’s milk, or are unrelated to cow’s milk exposure is challenging yet essential for effective management. While the clinical presentation of non-IgE-mediated CMPA can vary, this condition is usually self-limited and resolves by 1 to 6 years of age. Food antigen-specific immunoglobulin G (IgG) panels that are not evidence-based should be avoided because they can lead to overdiagnosis of presumed food intolerances. Overdiagnosis of CMPA can result in overuse of extensively hydrolyzed formulas and have significant cost implications for families. This statement focuses on delayed non-IgE/cell-mediated CMPA and assists health care providers to distinguish between and identify varied reactions to cow’s milk, discusses the role of diagnostic testing, and provides management recommendations based on best evidence.
Keywords: Amino acid formula (AAF); Cow’s milk intolerance (CMI); Cow’s milk protein allergy (CMPA); Extensively hydrolyzed formula (eHF); Food protein-induced allergic proctocolitis (FPIAP); Food protein-induced enterocolitis syndrome (FPIES); Food protein-induced enteropathy (FPE); Oral food challenge (OFC).
INTRODUCTION AND DEFINITIONS
Cow’s milk ingestion can elicit reactions with varied symptoms that can overlap with clinical conditions unrelated to cow’s milk ingestion. Diagnosis of such reactions is further complicated by inconsistent use of terminology to describe them, leading to confusion among both clinicians and families. While this statement focuses on non-IgE-mediated cow’s milk protein allergy (CMPA), it is important to define frequently used terms such as cow’s milk intolerance (CMI), which is often confused with CMPA and its various sub-types.
Cow’s milk intolerance (CMI)
CMI is a non-immunologic adverse response to cow’s milk due to enzymatic deficiency of lactase. Lactase deficiency is rare in infancy and usually develops during late childhood or into adulthood, with symptoms limited to the gastrointestinal tract. CMI is a benign condition that leads to inadequate digestion of lactose, resulting in lactose intolerance. Maldigestion and malabsorption of lactose causes fermentation of undigested lactose by colonic bacteria and the production of hydrogen, carbon dioxide, and lactic acid, which in turn causes abdominal pain, bloating, flatulence, and/or watery diarrhea. Severity depends on the quantity of lactose ingested relative to intestinal lactase activity. Lactose intolerance is treated by reducing cow’s milk intake or by using lactose hydrolyzing agents[1].
Cow’s milk protein allergy (CMPA)
CMPA refers to immune-mediated adverse reactions to one or more proteins in cow’s milk[2][3], and is primarily seen in childhood. CMPA can be immediate (IgE-mediated), delayed (non-IgE- or cell-mediated) or mixed (IgE- and non-IgE/cell-mediated) (Figure 1)[4]-[6]. Clinical presentations are variable and can involve multiple organ systems including the GI tract, respiratory system, and skin. Reaction severity can range from mild or moderate to severe or life-threatening.
Cow’s milk proteins are comprised of two major protein fractions: casein (76% to 86%), and whey (14% to 24%), which is made up of beta-lactoglobulin, alpha-lactalbumin, serum albumin, and serum immunoglobulins. Of these, casein and beta-lactoglobulin are the two most allergenic and heat-resistant proteins, with individuals having variable degrees of sensitivity to each[1]. Ingested milk proteins are degraded by gastric acid and luminal digestive enzymes. When the intestinal mucosa is exposed to these cow’s milk antigens, antigen-presenting cells (APCs) interact with T and B lymphocytes. In CMPA, activated T and B cells of lymphoid follicles migrate through the lymphatic system and blood vessels to different organs, causing an inflammatory reaction in the target organ, increased intestinal permeability, and clinical manifestations.
Classification of adverse reactions to cow’s milk (Figure 1)
- IgE-mediated (immediate): Anaphylactic reaction involving multiple systems including urticaria, angioedema of lips and eyes, vomiting, wheezing[4], or hypotension.
- Non-IgE/cell-mediated (delayed): Food-specific IgE is typically absent and GI reaction after exposure to food is delayed, and may have a chronic presentation[5].
a) Food protein-induced allergic proctocolitis (FPIAP) - rectal bleed +/– mucus
b) Food protein-induced enteropathy (FPE) - diarrhea and failure to thrive
c) Food protein-induced enterocolitis syndrome (FPIES) - diarrhea, vomiting, and lethargy
3. Mixed IgE and non-IgE/cell-mediated (delayed): Symptoms are delayed and depend on extent of eosinophilic infiltration of affected organs[6].
a) Eosinophilic esophagitis (EoE) - vomiting or dysphagia
b) Allergic eosinophilic gastrointestinal diseases (EGIDs) - diarrhea +/– rectal bleeding
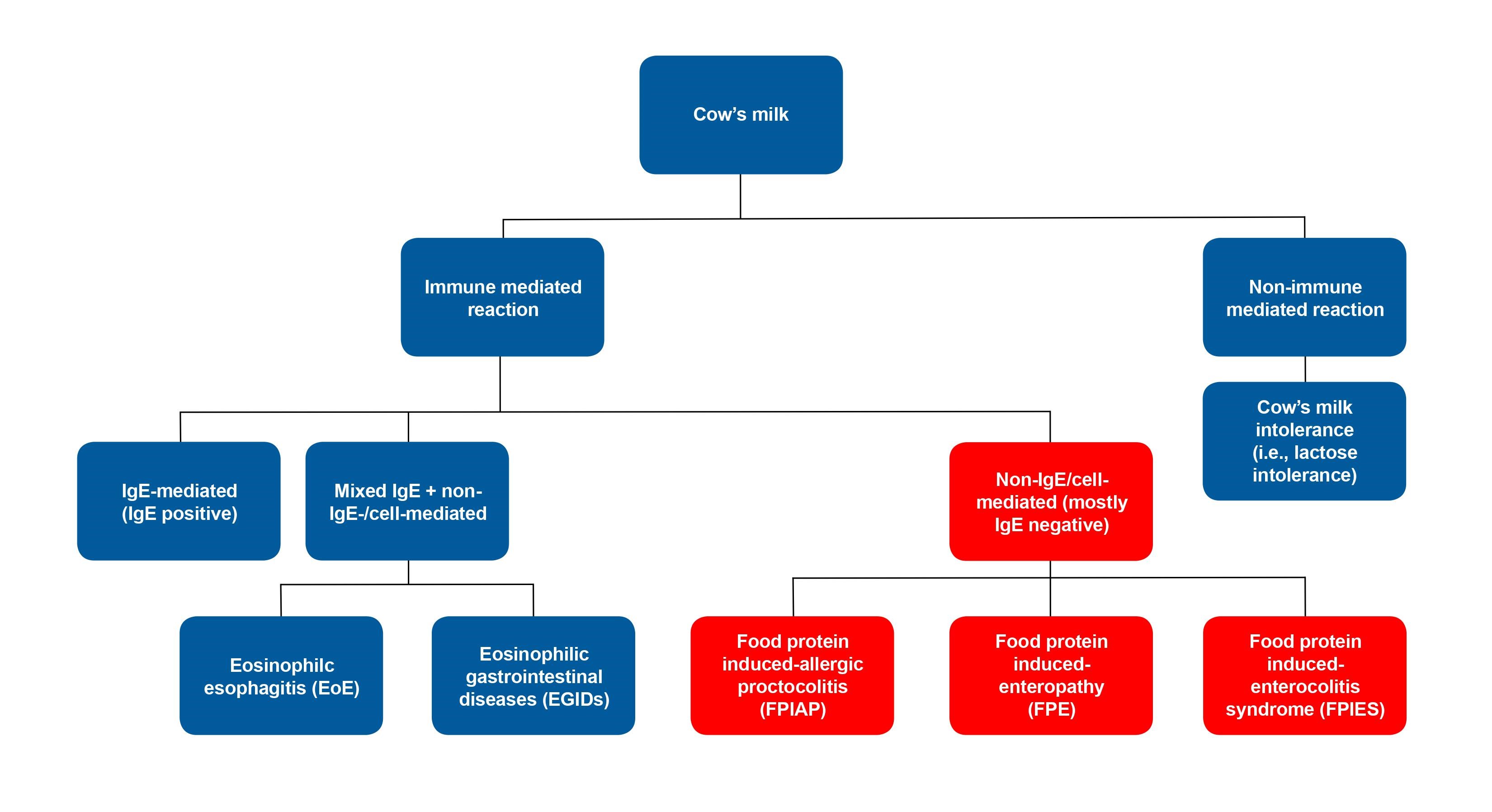
Figure 1. Classification of adverse reactions to cow’s milk
This statement focuses on non-IgE/cell-mediated CMPA. IgE-mediated and mixed IgE/non-IgE mediated reactions are beyond the scope of this statement.
EPIDEMIOLOGY
Cow’s milk protein (CMP) is the leading cause of allergy in infants and children younger than 3 years of age, but the reported prevalence of CMPA varies widely by population studied. While 5% to 15% of infants show symptoms suggesting adverse reactions to cow’s milk protein[7], the actual prevalence estimates of CMPA vary from 2% to 7.5%[2]. Population-based studies report a prevalence ranging between 1.9% and 4.9% in young children[8], and falls to <1% in children older than 6 years of age. The incidence of CMPA in exclusively breastfed infants is reported to be 0.4% to 0.5%[9][10].
The reported prevalence of CMPA sub-types also varies widely. A large Israeli birth cohort reported a FPIAP prevalence of 0.16%[11]. By contrast, a cumulative incidence of 17% was reported in a recent prospective observational study of healthy newborns with occult blood diagnosed as FPIAP by paediatricians in the United States, leading to an overdiagnosis[12]. Food protein-induced enteropathy (FPE) is relatively uncommon and obscure[13], with one Finnish study reporting a prevalence of FPE to cow’s milk in older children of 2.2%[14].
Food protein-induced allergic enterocolitis syndrome (FPIES) was recognized and formally defined in the mid-1970s[15] and is now regarded as a rare disease with a cumulative incidence ranging from 0.015% to 0.7% in children[16]-[18]. The incidence of FPIES in Australian infants (<24 months of age) was 15.4/100,000/year[17]. In a prospective single-hospital birth cohort over 2 years, a cumulative incidence of infants with cow’s milk-induced FPIES of 3/1000 newborns (0.34%) was reported[18][19].
Co-atopic disease affects 40% to 60% of children with FPIES[16][20][21], and 40% to 50% of children with FPE and FPIAP[12][14]. Both FPE and FPIES have been reported in infants with Down syndrome, who may present with a protracted course[22] due to intrinsic immune defects[23].
EVALUATION OF INFANTS AND CHILDREN WITH SUSPECTED NON-IgE-MEDIATED CMPA
Non-IgE-mediated CMPA is a spectrum of disease in infancy that manifests with delayed gastrointestinal symptoms and consists of three main entities − FPIAP, FPE and FPIES[5] − each with some distinctive features[3]. FPIAP and FPIES are at opposite ends of the severity spectrum. Symptoms may be mild, moderate, or severe, and involve the gastrointestinal system primarily, with potential risk for poor weight gain due to chronic diarrhea, vomiting, refusal to feed, and rectal bleeding. Cutaneous and respiratory system involvement may be seen in children with atypical FPIES. The most common triggers are cow’s milk proteins or soy, although eggs, wheat, rice, and other foods can also be triggers[24][25].
Exclusively breastfed infants with non-IgE-mediated CMPA tend to have mild to moderate symptoms[7], and severe or life-threatening symptoms are rare in this population[26]. Non-IgE-mediated CMPA is essentially a clinical diagnosis, with the exception of FPE, which requires histological confirmation. Symptom resolution is noted on avoidance of the implicated food. Taking a careful history, including the atopic history of the infant or child and family and a thorough physical exam, are key to elucidating different phenotypic presentations and differentiating conditions unrelated to cow’s milk exposure. A family history of atopy is present in 60% of first-degree relatives in FPIAP and in 80% of first-degree relatives in FPIES cases[16][17][27].
Most infants or children with non-IgE-mediated CMPA have a negative IgE and are unlikely to develop multiple food allergies[28]. However, a few children with atopic dermatitis, asthma, or rhino conjunctivitis are at increased risk for developing IgE-positive CMPA (e.g., atypical FPIES). Due to the lack of diagnostic tests for non-IgE-mediated CMPA and its non-specific symptoms that overlap with a wide variety of other conditions, it is important to consider both the accuracy of diagnosis and weigh the benefits and risks of interventions.
Food protein-induced allergic proctocolitis (FPIAP)
FPIAP can be seen in breastfed and formula-fed infants. One recent meta-analysis has reported that up to 50% of infants with FPIAP are breastfed[29][30]. Symptoms such as streaks of blood in stool or hematochezia may develop in the first 2 to 8 weeks of life, induced by localized inflammation of the distal colon in otherwise healthy, well-appearing infants [27][29]. A dense eosinophilic infiltration of the rectosigmoid mucosa is present, although it is unclear why the inflammatory response is localized to the distal colon[27].
FPIAP in infancy does not require laboratory work-up, although anemia, peripheral eosinophilia, and hypoalbuminemia may be present[31][32]. Response to the dietary elimination of dairy and soy is the only diagnostic test required because IgE and food-specific radioallergosorbent test (RAST) are often negative.
Most infants with FPIAP will have progressive resolution of symptoms within 3 days of removing the offending protein from the maternal diet or changing to a hypoallergenic formula, although complete resolution may take up to 2 weeks[29][33]. Colonoscopy with biopsy is not performed, but may be indicated if there is diagnostic confusion.
Food protein-induced enteropathy (FPE)
FPE predominantly affects the small intestine, leading to malabsorption and failure to thrive[5]. FPE is characterized by diarrhea in infancy, often with emesis and abdominal distension[31][32]. Cow’s milk-specific T-cell infiltration (cytotoxic CD8+ T cells in particular) of the jejunum causes malabsorption[27]. Distinguishing features include malabsorption with steatorrhea in up to 80% of infants and a lack of the acute symptoms seen in FPIES[13].
FPE requires laboratory tests and gastroscopy with biopsy to confirm diagnosis and to differentiate this condition from other possible causes of failure to thrive and diarrhea in infancy (e.g., infection, celiac disease, and pancreatic insufficiency in cystic fibrosis). Laboratory tests can show peripheral eosinophilia, fat-soluble vitamin deficiency, anemia, hypoproteinemia, and prolonged coagulation time[13][34][35]. Stool tests can show fat malabsorption in 80% of patients[13]. Jejunal biopsies show villous atrophy and crypt hyperplasia.
Food protein-induced enterocolitis syndrome (FPIES)
FPIES caused by cow’s milk and soy is rare in exclusively breastfed infants, although it has been noted in some case reports[36][37]. Infants and young children with FPIES present with symptoms[34][38] that vary in severity but are characterized by repetitive vomiting, watery and sometimes bloody diarrhea, pallor, and lethargy[39]. Food-specific T-cell infiltration and inflammation cause increased intestinal permeability, leading to fluid shifts into the GI tract with increasing antigen influx[27]. An estimated 15% of infants present with severe symptoms including dehydration, hypovolemic shock, and metabolic acidosis, which can be mistaken for infectious enteritis or sepsis. The timing and severity of symptoms, age of onset, and food triggers can also vary[27].
The most frequently incriminated foods for FPIES in the United States and Europe are cow’s milk, soy, and grains[40][41], with other implicated foods being rice, chicken, meat, fruits, corn, wheat, white and sweet potatoes, and mushrooms[39][42][43]. Other foods such as oats, eggs, seafood (fish and shellfish) are common triggers in older children[44].
Acute FPIES caused by cow’s milk proteins or soy presents in infants 2 to 7 months of age with onset of symptoms occuring within 1 to 4 hours after ingesting the offending food[45][46]. The hallmark symptom in over 95% of cases is profuse, repetitive vomiting, which may also be associated with pallor, lethargy, and diarrhea in 25% to 50% of infants after 5 to 10 hours. In 15% of infants, symptoms are severe and can progress to hypovolemic shock, hypothermia, methemoglobinemia, and acidemia, resulting in a sepsis-like picture. However, these symptoms usually resolve within 24 hours of eliminating the trigger food[46].
Chronic FPIES caused by cow’s milk proteins or soy can have a more insidious onset in some formula-fed infants younger than 4 months of age, with daily ingestion of the inciting food protein causing delayed symptoms up to 24 hours after ingestion. Symptoms include intermittent emesis, chronic watery diarrhea, and failure to thrive[15]. Severe symptoms can include dehydration and shock, lethargy, cyanosis, hypotension, hypothermia, and methemoglobinemia[26], which is under-recognized as a possible manifestation of FPIES[47]. Symptoms improve within 3 to 10 days of removing the inciting formula and introducing a hypoallergenic formula[46].
In atypical FPIES, some children with FPIES may have a positive specific IgE to the causal protein[48][49]. These children appear to be at increased risk for developing IgE-mediated allergy, and have a more protracted course[34][45].
FPIES can be challenging to diagnose because it is an unfamiliar condition with non-specific symptoms that can mimic viral gastroenteritis, anaphylaxis, or sepsis. FPIES is a clinical diagnosis, with a careful history being the most important diagnostic tool. More recent evidence-based diagnostic criteria emphasize that repetitive vomiting (a major criteria) is the cardinal feature of acute FPIES, with at least three minor criteria required for diagnosis[46]. Differential diagnosis includes sepsis, infectious gastroenteritis, necrotizing enterocolitis, bowel obstruction, inborn errors of metabolism, celiac disease, inflammatory bowel disease, eosinophilic gastrointestinal disease, and anaphylaxis[21][50].
There are no specific diagnostic tests for FPIES, but the ill-appearing infant warrants investigations that includes complete blood count (CBC), blood gas, and chemistry to detect anemia, leukocytosis with neutrophilia, eosinophilia, thrombocytosis, non-anion gap acidosis, methemoglobinemia, and hypoalbuminemia[39]. Laboratory work-up is generally normal after the FPIES reaction has resolved. Some cases of FPIES have concomitant IgE sensitization to certain foods, and this is referred to as ‘atypical FPIES’[51]. However, most children with FPIES have a negative skin prick test and negative food-specific IgE. An oral food challenge (OFC) under medical supervision is the gold standard for diagnosis but is not recommended when the clinical history is typical[46][52]. However, an OFC may be helpful in identifying the child who has outgrown FPIES.
Alternative but unproven diagnostic investigations (e.g., antigen-specific IgG food panel testing) should be avoided because they are not evidence-based and can lead to overdiagnosis of presumed food intolerances[53][54].
MANAGEMENT OF INFANTS AND CHILDREN WITH NON-IgE-MEDIATED CMPA
There is evidence-based guidance for use of dietary products to treat non-IgE-mediated CMPA[55]-[59], and practical guidelines to assist in effective management of infants and children with CMPA[8][25][60][61]. Strict avoidance of CMP is the safest strategy. Both the need for and best choice of infant formula depend on the age of the child and the presence of other food allergies. Most infants and children with CMPA tolerate an extensively hydrolyzed formula (eHF), with <10% of infants requiring an amino acid formula (AAF)[62].
The exact mechanism involved in tolerance development remains unclear[7]. The natural history of non-IgE-mediated gastrointestinal food allergies is generally favourable, with most affected infants achieving tolerance in the first years of life and most cases resolving before school age[30]. Tolerance is achieved in 50% of children by 1 year of age, greater than 75% by age 3 years, and greater than 90% by age 6 years[63]. Prolonged dietary eliminations should be avoided because this can impair quality of life and growth while incurring unnecessary costs for families.
For FPIAP in breastfed infants, empiric elimination of dairy and soy, the most common allergens from the maternal diet, should be considered for 2 weeks[64]. Other possible triggers include egg and corn, which can be eliminated from maternal diet if symptoms do not resolve with dairy and soy elimination[39]. Counselling by a qualified paediatric dietitian is strongly recommended to ensure nutritional needs of the infant are met and to avoid hidden sources of allergens. Supporting breastfeeding mothers, and supplementing their diet with calcium 500 mg twice daily and vitamin D (based on geographic location and nutritional assessment of maternal diet) are important. If an infant’s symptoms persist, an eHF is recommended first, with AAF being reserved for the less than 10% of infants who do not respond to eHF.
For FPIAP in formula-fed infants, an elimination diet usually starts with an eHF with proven efficacy in infants with CMPA[8][56], although AAF may be indicated for some infants whose symptoms persist beyond 2 to 4 weeks. Alternative animal milk sources (e.g., goat’s or sheep’s milk) must be strictly avoided due to high cross-reactivity and nutritional inadequacy[56][65][66]. Soy-based formula is an option for infants older than 6 months old if eHF is not accepted or tolerated, provided tolerance to soy protein has been established[60]. A meta-analysis of probiotic supplementation did not show earlier resolution of hematochezia, but was associated with a higher rate of tolerance to CMP at the end of 3 years[67].
FPIAP symptoms typically improve within 72 hours of food elimination, although stools may take up to 2 weeks to normalize. Most infants tolerate CMP by age 1 year and the condition rarely persists beyond age 2 years[12][31]. Re-challenge at home with fresh pasteurized cow’s milk may be used to assess for tolerance to CMP after 1 year of age, with most children developing tolerance by 1 to 2 years of age[68]-[70].
For FPE in breastfed infants with failure to thrive, consider empiric elimination of cow’s milk and soy from the maternal diet for 2 to 4 weeks. If symptoms do not improve OR if the infant is formula-fed, an AAF is recommended[32]. It is important to monitor for appropriate growth and nutritional deficiencies.
FPE symptoms take weeks to resolve after eliminating the offending protein because villous injury and malabsorption can be significant. Re-challenge at home with fresh pasteurized cow’s milk to assess for tolerance to CMP after age 1 year is recommended, as most children develop tolerance by age 1 to 2 years[68]-[70]. If tolerance is not achieved at age 1 year, a rechallenge at home may be considered at age 18 months. Heath care providers can assure parents that the long-term prognosis is good, and tolerance to the offending protein is achieved in most infants by 2 to 3 years of age[24].
For FPIES, there are recent international consensus guidelines for diagnosis and management[46][52]. Acute FPIES is a medical emergency that requires treatment with aggressive fluid resuscitation, steroids, and ondansetron (with potential risk for prolonged QT interval) in infants with hypovolemic shock[39].
In breastfed infants, symptomatic FPIES is rare. Mild to moderate acute FPIES can resolve with oral rehydration or breastfeeding at home. If an infant reacts to breastfeeding, maternal elimination of dairy and soy is recommended. If symptoms do not resolve, breastfeeding should be discontinued and eHF is recommended. Avoid recurrence of acute relapse by strictly avoiding offending food protein(s).
In formula-fed infants with FPIES, the World Allergy Organization recommends the use of eHF[8], with 10% to 20% of infants requiring AAF[29][41]. Both the American Academy of Pediatrics (AAP) [55] and the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN)[60] recommend AAF for infants with FPIES who are failing to thrive[8][46][71]. Strict avoidance of all dairy, including heated or baked forms of the offending allergen, is recommended. A reasonable approach is to start with eHF and consider switching to AAF if there is no relief of symptoms or resumption of growth within 2 weeks.
Acute FPIES generally resolves in a few hours with rehydration, whereas chronic FPIES takes a few days to 2 weeks to resolve. Most children outgrow FPIES. The overall rate of resolution ranges from 50% to 90% by 6 years of age[29][41], with rates varying by type of food and geographic location[72][73]. A retrospective series from the US reported resolution rates of FPIES related to cow’s milk or soy of 35% by age 2 years, 70% by age 3 years, and 85% by age 5 years[41]. Infants with atypical FPIES and concomitant IgE sensitization generally have a more protracted course[20] and are at risk for developing IgE-mediated food allergy[41].
An OFC should be performed under close physician supervision 12 to 18 months after the last reaction to assess for resolution of FPIES[46][74]. Follow-up to avoid unnecessary food restrictions and nutritional deficiencies is important.
In summary, the non-specific symptoms of non-IgE-mediated CMPA and lack of specific testing modalities make diagnosis challenging. However, being familiar with the three sub-types of this condition and more recent diagnostic criteria for FPIES can aid identification and management. Considering the risks of under- and overdiagnosis of CMPA should be part of the clinical calculus. Premature discontinuation of breastfeeding, overuse of expensive eHF or AAF, and parental fear of allergic reactions are consequences that are not easily undone. Physicians can help mitigate such risks by offering thoughtful explanations and support to parents and caregivers, while considering collaborative treatment planning.
RECOMMENDATIONS:
- Using appropriate terminology consistently with families is important. Cow’s milk protein allergy (CMPA) should not be confused with cow’s milk intolerance (CMI), which is rare in infancy.
- Food protein-induced allergic proctocolitis (FPIAP) is the most common subtype of non-IgE-mediated CMPA in breastfed and formula-fed infants. Exercise prudence to avoid overdiagnosis of CMPA and consequent overuse of hydrolyzed formulas, which can have significant cost implications for some families.
- Avoid prolonged dietary eliminations when managing non-IgE-mediated food allergies, as there are nutritional risks associated with this approach.
- There are no specific diagnostic tests for non-IgE-mediated CMPA, except for small bowel biopsy for confirmation of food protein-induced enteropathy (FPE). Avoid using unproven diagnostic tests (i.e., panels of food antigen-specific IgG), which can lead to overdiagnosis of presumed food intolerances.
- When unsure of diagnosis, refer infants to a paediatric gastroenterologist to avoid overuse of hydrolyzed formulas.
- For breastfed infants with FPIAP, encourage breastfeeding while eliminating dairy and soy from maternal diet. A dietitian referral can help identify hidden sources of allergens and ensure maternal nutritional needs are met. If symptoms persist, consider using extensively hydrolyzed formula (eHF).
- For formula-fed infants with CMPA, eHF must be considered. For severe FPIES with failure to thrive, feeding with amino acid formula is recommended.
- For FPIAP and FPE, consider re-challenging with fresh pasteurized cow’s milk at home after 1 year of age.
- For food protein-induced enterocolitis syndrome (FPIES), an oral food challenge should be performed under close physician supervision 12 to 18 months after the last reaction to assess for resolution.
Acknowledgements
This position statement has been reviewed by the Allergy Section Executive and Community Paediatrics Committee of the Canadian Paediatric Society.
CANADIAN PAEDIATRIC SOCIETY NUTRITION AND GASTROENTEROLOGY COMMITTEE (March 2022)
Members: Belal Alshaikh MD, Pushpa Sathya MD, Gina Rempel MD, Ana Sant’Anna MD (Chair), Rilla Schneider MD (Resident Member), Christopher Tomlinson MD (Past Member), Linda Casey MD (Past Member), Eddy Lau MD (Board Representative)
Liaisons: Subhadeep Chakrabarti (Health Canada), Jennifer McCrea (Health Canada), Tanis R. Fenton RD, PhD (Dietitians of Canada), Laura N. Haiek MD, MSc (Breastfeeding Committee for Canada)
Prinicipal authors: Pushpa Sathya MD; Tanis R. Fenton RD, PhD
References
- Bahna SL. Cow’s milk allergy versus cow’s milk intolerance. Ann Allergy Asthma Immunol 2002;89(6 Suppl 1):56-60. doi: 10.1016/s1081-1206(10)62124-2.
- Hill DJ, Firer MA, Shelton MJ, Hosking CS. Manifestations of milk allergy in infancy: Clinical and immunologic findings. J Pediatr 1986;109(2):270-76. doi: 10.1016/s0022-3476(86)80384-5.
- Tordesillas L, Berin MC, Sampson HA. Immunology of food allergy. Immunity 2017;47(1):32-50. doi: 10.1016/j.immuni.2017.07.004.
- Waserman S, Bégin P, Watson W. IgE-mediated food allergy. Allergy Asthma Clin Immunol 2018;14(Suppl 2):55. doi: 10.1186/s13223-018-0284-3.
- Caubet JC, Szajewska H, Shamir R, Nowak-Węgrzyn A. Non-IgE-mediated gastrointestinal food allergies in children. Pediatr Allergy Immunol 2017;28(1):6-17. doi: 10.1111/pai.12659.
- Koutri E, Papadopoulou A. Eosinophilic gastrointestinal diseases in childhood. Ann Nutr Metab 2018;73(Suppl 4):18-28. doi: 10.1159/000493668.
- Host A. Frequency of cow’s milk allergy in childhood. Ann Allergy Asthma Immunol 2002;89(6 Suppl 1):33-37. doi: 10.1016/s1081-1206(10)62120-5.
- Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) guidelines. World Allergy Organ J 2010;3(4):57-161. doi: 10.1097/WOX.0b013e3181defeb9.
- Jakobsson I, Lindberg T. A prospective study of cow’s milk intolerance in Swedish infants. Acta Pediatr Scand 1979;68(6):853-59. doi: 10.1111/j.1651-2227.1979.tb08223.x.
- Saarinen KM, Juntunen-Badman K, Järvenpää AL, et al. Supplementary feeding in maternity hospitals and the risk of cow’s milk allergy: A prospective study of 6209 infants. J Allergy Clin Immunol 1999;104(2 Pt 1):457-61. doi: 10.1016/s0091-6749(99)70393-3.
- Elizur A, Cohen M, Goldberg MR, et al. Cow’s milk associated rectal bleeding: A population based prospective study. Pediatr Allergy Immunol 2012;23(8):766-70. doi: 10.1111/pai.12009.
- Martin VM, Virkud YV, Seay H, et al. Prospective assessment of pediatrician-diagnosed food protein-induced allergic proctocolitis by gross or occult blood. J Allergy Clin Immunol Pract 2020;8(5):1692-99.e1. doi: 10.1016/j.jaip.2019.12.029.
- Savilahti E. Food induced malabsorption syndromes. J Pediatr Gastroenterol Nutr 2000;30 Suppl):S61-6. doi: 10.1097/00005176-200001001-00010.
- Kokkonen J, Haapalahti M, Tikkanen S, Karttunen R, Savilahti E. Gastrointestinal complaints and diagnosis in children: A population-based study. Acta Pediatr 2004;93(7):880-86.
- Powell GK. Milk- and soy-induced enterocolitis of infancy. Clinical features and standardization of challenge. J Pediatr 1978;93(4):553-60. doi: 10.1016/s0022-3476(78)80887-7.
- Nowak-Wegrzyn A, Warren CM, Brown-Whitehorn T, Cianferoni A, Schultz-Matney F, Gupta RS. Food protein-induced enterocolitis syndrome in the US population-based study. J Allergy Clin Immunol 2019;144(4):1128-30. doi: 10.1016/j.jaci.2019.06.032.
- Mehr S, Frith K, et al. Food protein-induced enterocolitis syndrome in Australia: A population-based study, 2012-2014. J Allergy Clin Immunol 2017;140(5):1323-30. doi: 10.1016/j.jaci.2017.03.027.
- Alonso SB, Ezquiaga JG, Berzal PT, et al. Food protein-induced allergic enterocolitis syndrome: Increased prevalence of the great unknown—results of the PREVALE study. J Allergy Clin Immunol 2019;143(1):430-33. doi: 10.1016/j.jaci.2018.08.045.
- Katz Y, Goldberg MR, Rajuan N, Cohen A, Leshno M. The prevalence and natural course of food protein-induced enterocolitis syndrome to cow’s milk: A large-scale, prospective population-based study. J Allergy Clin Immunol 2011;127(3):647-53.e1-3. doi: 10.1016/j.jaci.2010.12.1105.
- Maciag MC, Bartnikas LM, Schirer SH, et al. A slice of food protein-induced enterocolitis syndrome (FPIES): Insights from 441 children with FPIES as provided by caregivers in the International FPIES Association. J Allergy Clin Immunol Pract 2020;8(5):1702-09. doi: 10.1016/j.jaip.2020.01.030.
- Barasche J, Stollar F, Bergmann MM, Caubet JC. Severely altered-consciousness status and profuse vomiting in infants: Food protein-induced enterocolitis syndrome (FPIES), a challenging diagnosis. Pediatr Emerg Care 2018;34(10):e187-89. doi: 10.1097/PEC.0000000000000921.
- Wakiguchi H, Hasegawa S, Kaneyasu H, et al. Long-lasting non-IgE-mediated gastrointestinal cow’s milk allergy in infants with Down syndrome. Pediatr Allergy Immunol 2015;26(8):821-23. doi: 10.1111/pai.12351.
- Kusters MAA, Verstegen RHJ, Gemen EFA, de Vries E. Intrinsic defect of the immune system in children with Down syndrome: A review. Clin Exp Immunol 2009;156(2):189-93. doi: 10.1111/j.1365-2249.2009.03890.x.
- Feuille E, Nowak-Węgrzyn. Food protein-induced enterocolitis syndrome, allergic proctocolitis, and enteropathy. Curr Allergy Asthma Rep 2015;15(8):50. doi: 10.1007/s11882-015-0546-9.
- Abrams EM, Hildebrand KJ, Chan ES. Non-IgE-mediated food allergy: Evaluation and management. Pediatr Child Health 2021;26(3):173-81. doi: 10.1093/pch/pxaa131.
- De Greef E, Hauser B, Devreker T, Veereman-Wauters G, Vandenplas Y. Diagnosis and management of cow’s milk protein allergy in infants. World J Pediatr 2012;8(1):19-24. doi: 10.1007/s12519-012-0332-x.
- Labrosse R, Graham F, Caubet JC. Non-IgE-mediated gastrointestinal food allergies in children: An update. Nutrients 2020;12(7):2086. doi: 10.3390/nu12072086.
- Saarinen KM, Pelkonen AS, Mäkelä MJ, Savilahti E. Clinical course and prognosis of cow’s milk allergy are dependent on milk-specific IgE status. J Allergy Clin Immunol 2005;116(4):869-75. doi: 10.1016/j.jaci.2005.06.018.
- Lake AM, Whitington PF, Hamilton SR. Dietary protein-induced colitis in breast-fed infants. J Pediatr 1982;101(6):906-10. doi: 10.1016/s0022-3476(82)80008-5.
- Lozinsky AC, de Morais MB. Eosinophilic colitis in infants. J Pediatr (Rio J) 2014;90(1):16-21. doi: 10.1016/j.jped.2013.03.024.
- Kuitunen P, Visakorpi JK, Savilahti E, Pelkonen P. Malabsorption syndrome with cow’s milk intolerance. Clinical findings and course in 54 cases. Arch Dis Child 1975;50(5):351-56. doi: 10.1136/adc.50.5.351.
- Iyngkaran N, Robinson MJ, Prathap K, Sumithran E, Yadav M. Cow’s milk protein-sensitive enteropathy. Combined clinical and histological criteria for diagnosis. Arch Dis Child 1978;53(1):20-26. doi: 10.1136/adc.53.1.20.
- Lake AM. Food-induced eosinophilic proctocolitis. J Pediatr Gastroenterol Nutr 2000;30 (Suppl):S58-60. doi: 10.1097/00005176-200001001-00009.
- Caubet JC, Ford LS, Sickles L, et al. Clinical features and resolution of food protein-induced enterocolitis syndrome: 10-year experience. J Allergy Clin Immunol 2014;34(2):382-89. doi: 10.1016/j.jaci.2014.04.008.
- Boyce JA, Assa’ad AN, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: Summary of the NAIAD-sponsored expert panel report. J Allergy Clin Immunol 2010;126(6):1105-18. doi: 10.1016/j.jaci.2010.10.008.
- Tan J, Campbell D, Mehr S. Food protein-induced enterocolitis syndrome in an exclusively breast-fed infant – an uncommon entity. J Allergy Clin Immunol 2012;129(3):873-74. doi: 10.1016/j.jaci.2011.12.1000.
- Baldo F, Bevacqua M, Corrado D, et al. FPIES in exclusively breastfed infants: Two case reports and review of the literature. Ital J Pediatr 2020;46(1):144. doi: 10.1186/s13052-020-00910-8.
- Michelet M, Schluckebier D, Petit LM, Caubet JC. Food protein-induced enterocolitis syndrome – A review of the literature with focus on clinical management. J Asthma Allergy 2017:10:197-207. doi: 10.2147/JAA.S100379.
- Mehr S, Kakakios A, Frith K, Kemp AS. Food protein-induced enterocolitis syndrome: 16-year experience. Pediatrics 2009;123(3):e459-64. doi: 10.1542/peds.2008-2029.
- Levy Y, Danon YL. Food protein-induced enterocolitis syndrome – not only due to cow’s milk and soy. Pediatr Allergy Immunol 2003;14(4):325-29. doi: 10.1034/j.1399-3038.2003.00039.x.
- Wang KY, Lee J, Cianferoni A, et al. Food protein-induced enterocolitis syndrome food challenges: Experience from a large referral centre. J Allergy Clin Immunol Pract 2019;7(2):444-50. doi: 10.1016/j.jaip.2018.09.009.
- Mehr S, Kakakios AM, Kemp AS. Rice: A common and severe cause of food protein-induced enterocolitis syndrome. Arch Dis Child 2009;94(3):220-23. doi: 10.1136/adc.2008.145144.
- Katz Y, Goldberg MR. Natural history of food protein-induced enterocolitis syndrome. Curr Opin Allergy Clin Immunol 2014; 14(3):229-39. doi: 10.1097/ACI.0000000000000053.
- Garcia MR, Jimenez Diaz F. Food protein-induced enterocolitis syndrome (FPIES): Our experience. J Allergy Clin Immunol 2012;129(2 Suppl):AB34.
- Nowak-Węgrzyn A, Katz Y, Mehr SS, Koletzko S. Non-IgE-mediated gastrointestinal food allergy. J Allergy Clin Immunol 2015;135(5):1114-24. doi: 10.1016/j.jaci.2015.03.025.
- Nowak-Węgrzyn A, Chehade M, Groetch ME, et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: Executive summary—Workgroup report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 2017;139(4):1111-26.e4. doi: 10.1016/j.jaci.2016.12.966.
- Feuille E, Menon NR, Huang F, Greenhawt M, Nowak-Węgrzyn A. Knowledge of food protein-induced enterocolitis syndrome among general pediatricians. J Allergy Clin Immunol 2017;119(3):291-92.e.3. doi: 10.1016/j.anai.2017.07.001.
- Nowak-Węgrzyn A, Sampson HA, Wood RA, Sicherer SH. Food protein-induced enterocolitis syndrome caused by solid food proteins. Pediatrics 2003;111(4 Pt 1):829-35. doi: 10.1542/peds.111.4.829.
- Banzato C, Piacentini GL, Comberiati P, Mazzei F, Boner AL, Peroni DG. Unusual shift from IgE-mediated milk allergy to food protein-induced enterocolitis syndrome. Eur Ann Allergy Clin Immunol 2013;45(6):209-11.
- Fiocchi A, Claps A, Dahdah L, Brindisi G, Dionisi-Vici C, Martelli A. Differential diagnosis of food protein-induced enterocolitis syndrome. Curr Opin Allergy Clin Immunol 2014;14(3):246-54. doi: 10.1097/ACI.0000000000000057.
- Sicherer SH, Eigenmann PA, Sampson HA. Clinical features of food protein-induced enterocolitis syndrome. J Pediatr 1998; 133(2):214-19. doi: 10.1016/s0022-3476(98)70222-7.
- Cherian S, Varshney P. Food protein-induced enterocolitis syndrome (FPIES): Review of recent guidelines. Curr Allergy Asthma Rep 2018;18(4):28. doi: 10.1007/s11882-018-0767-9.
- Kelso JM. Unproven diagnostic tests for adverse reactions to foods. J Allergy Clin Immunol Pract 2018;6(2):362-65. doi: 10.1016/j.jaip.2017.08.021.
- Myszkowska D, Zapata B, Bulanda M, Czarnobilska E. Non-IgE mediated hypersensitivity to food products or food intolerance—Problems of appropriate diagnostics. Medicina (Kaunas) 2021;57(11):1245. doi: 10.3390/medicina57111245.
- American Academy of Pediatrics, Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000;106(2 Pt1):346-49.
- Høst A, Koletzko B, Dreborg S, et al. Dietary products used in infants for treatment and prevention of food allergy. Joint statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch Dis Child 1999;81(1):80-84. doi: 10.1136/adc.81.1.80.
- Fenton MJ. Guidelines for the diagnosis and management of food allergy in the United States. Clin Transl Allergy 2011;1(S1):S10. doi:10.1186/2045-7022-1-S1-S10.
- Sampson HA, Aceves S, Bock SA, et al. Food allergy: A practice parameter update—2014. J Allergy Clin Immunol 2014;134(5):1016-25.e43. doi: 10.1016/j.jaci.2014.05.013.
- Dupont C, Chouraqui JP, de Boissieu D, et al. Dietary treatment of cows’ milk protein allergy in childhood: A commentary by the Committee on Nutrition of the French Society of Pediatrics. Br J Nutr 2012;107(3):325-38. doi: 10.1017/S0007114511004831.
- Koletzko S, Niggemann B, Arato A, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr 2012;55(2):221-29. doi: 10.1097/MPG.0b013e31825c9482.
- Vandenplas Y, Koletzko S, Isolauri E, et al. Guidelines for the diagnosis and management of cow’s milk protein allergy in infants. Arch Dis Child 2007;92(10):902-08. doi: 10.1136/adc.2006.110999.
- De Boissieu D, Dupont C. Allergy to extensively hydrolyzed cow’s milk proteins in infants: Safety and duration of amino acid-based formula. J Pediatr 2002;141(2):271-73. doi: 10.1067/mpd.2002.126299.
- Host A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol 2002;13 (Suppl 15):23-28. doi: 10.1034/j.1399-3038.13.s.15.7.x.
- Camargo LS, Da Silveira JAC, Taddei JA, Neto UF. Allergic proctocolitis in infants: Analysis of the evolution of nutritional status. Arq Gastroenterol 2016;53(4):262-66. doi: 10.1590/S0004-28032016000400010.
- Restani P. Gaiaschi A, Plebani A, et al. Cross-reactivity between milk proteins from different animal species. Clin Exp Allergy 1999;29(7):997-1004. doi: 10.1046/j.1365-2222.1999.00563.x.
- Spuergin P, Walter M, Schiltz E, Deichmann K, Forster J, Mueller H. Allergenicity of alpha-caseins from cow, sheep, and goat. Allergy 1997;52(3):293-98. doi: 10.1111/j.1398-9995.1997.tb00993.x.
- Qamar S, Deshmukh M, Patole S. Probiotics for cow’s milk protein allergy: A systematic review of randomized controlled trials. Eur J Pediatr 2019;178(8): 1139-49. doi: 10.1007/s00431-019-03397-6.
- Kokkonen J, Haapalahti M, Laurila K, Karttunen TJ, Mäki M. Cow’s milk protein-sensitive enteropathy at school age. J Pediatr 2001;139(6):797-803. doi: 10.1067/mpd.2001.118882.
- Kokkonen J, Tikkanen S, Savilahti E. Residual intestinal disease after milk allergy in infancy. J PediatrGastroenterol Nutr 2001;32(2):156-61. doi: 10.1097/00005176-200102000-00012.
- Walker WA. Cow’s milk protein-sensitive enteropathy at school age: A new entity or a spectrum of mucosal immune responses with age. J Pediatr 2001;139(6):765-66. doi: 10.1067/mpd.2001.120265.
- Venter C, Groetch M. Nutritional management of food protein-induced enterocolitis syndrome. Curr Opin Allergy Clin Immunol 2014;14(3):255-62. doi: 10.1097/ACI.0000000000000054.
- Nowak-Węgrzyn A, Assa’ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Work Group report: Oral food challenge testing. J Allergy Clin Immunol 2009;123(6 Suppl):S365-83. doi: 10.1016/j.jaci.2009.03.042.
- Caubet JC, Cianferoni A, Groetch M, Nowak-Węgrzyn A. Food protein-induced enterocolitis syndrome. Clin Exp Allergy 2019;49(9):1178-90. doi: 10.1111/cea.13415.
- Hill DJ, Murch SH, Rafferty K, Wallis P, Green CJ. The efficacy of amino acid-based formula in relieving the symptoms of cow’s milk allergy: A systematic review. Clin Exp Allergy 2007;37(6):808-22. doi: 10.1111/j.1365-2222.2007.02724.x.
Disclaimer: The recommendations in this position statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. Internet addresses are current at time of publication.
Last updated: Nov 13, 2024


